“Nothing in biology makes sense except in the light of evolution”
Theodosius Dobzhansky, 1973
There are upwards of 10 million new cancer cases a year in the world. One in three of us can expect that unwelcome diagnosis, and around one in four will succumb to metastatic, drug resistant disease. The big questions are, why are humans so vulnerable to cancer? what exactly is cancer as a biological process? and why is drug resistance the norm for advanced disease? Evolutionary biology has something to say about each of these grand challenges.
The Hallmarks of Cancer as evolutionary adaptations in a neoplasm[edit]
http://elledgelab.med.harvard.edu/?page_id=305
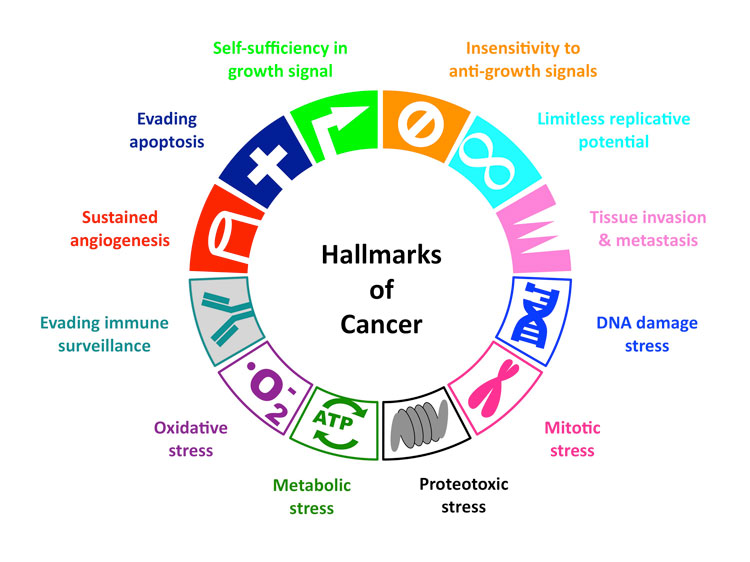
Hallmarks of Cancer
All cancer cells show 6 common traits (10 if you go more in-depth)
They are:
(1) Self-Sufficiency in growth signal, or in other words, they grow and multiply without being told to.
(3) Evading Apoptosis- they refuse to die, like zombies
(4) Limitless Reproduction Potential- They just keep multiplying!
(5) Sustained Angiogenesis- they keep making new blood vessels to feed themselves
(6) Tissue Invasion and Metastasis- they break away from their site of origin and invade surrounding tissues, eventually spreading (metastasizing) throughout the body….like an army of zombies.
Psychological stress can take a tremendous toll on your health. One of the reasons for this is because stress causes inflammation, which in turn is a hallmark of most diseases, from obesity and diabetes to heart disease and cancer.
Six years ago, I interviewed Donald (“Donnie”) Yance, an internationally known herbalist and nutritionist, who shared a really surprising piece of information: stress was actually pinned down as a cause of cancer all the way back in 1908. As Donnie said:
“Eli Jones, the great eclectic physician in cancer, and probably the most brilliant person that ever lived on the face of the planet, wrote a book in 1908 called “Cancer – Its Causes, Symptoms and Treatment.” There isn’t one inaccuracy I can find in that book, written more than 100 years ago.”
In this book, Dr. Jones revealed the top causes of cancer, and the No. 1 cause he listed was unresolved stress. Since then, a number of studies have confirmed this link. In the video above, two doctors at MD Anderson Cancer Center go into some of the details now known about stress and cancer.
Chronic Stress Makes Cancer Spread
Most recently, a study done on mice found that when the animals were chronically stressed, their lymphatic systems underwent changes that allowed cancer to spread more quickly and easily. As reported by Science Alert:1
“Although the study hasn’t been replicated in humans as yet, it’s a huge step towards understanding how stress – which has long been linked to cancer progression – actually helps tumour cells escape…
“Not for a minute are we suggesting that someone who’s just been diagnosed with cancer should not be stressed, because that would have to be one of the most stressful situations”… Erica Sloan from Monash University in Australia, told ABC News.2
“But rather how do we look after cancer patients, because this suggests that stress not only affects patient wellbeing but also gets into the body and affects how the tumour progresses.”
How Does Stress Promote the Spread of Cancer?
Cancer cells typically spread to other areas of the body either via your blood vessels, or through your lymphatic system. Stress hormones affect both of these pathways or channels. Here they were trying to determine how stress hormones affect the spread of cancer cells through the lymphatic system.
The mechanism they found is related to the way adrenaline activates the sympathetic nervous system (SNS) to increase the rate of lymph formation. Adrenaline also causes physical changes in the lymph vessels, allowing cancer cells to migrate into other body parts at a faster rate.
The National Cancer Institute has also previously stated that research with animal models suggests:3
“[Y]our body’s neuroendocrine response (release of hormones into your blood in response to stimulation of your nervous system) can directly alter important processes in cells that help protect against the formation of cancer, such as DNA repair and the regulation of cell growth.”
Other research4,5 has shown that the stress hormone norepinephrine may increase the growth rate of cancer.
Norepinephrine can stimulate tumor cells to produce two compounds (matrix metalloproteinases called MMP-2 and MMP-9) that break down the tissue around the tumor cells, thereby allowing the cells to more easily move into your bloodstream.
Once there, they can travel to other organs and tissues and form additional tumors.
Norepinephrine may also stimulate tumor cells to release a chemical (vascular endothelial growth factor, or VEGF) that aids the growth of the blood vessels that feed cancer cells. This too can increase the growth and spread of the cancer.
Epinephrine — yet another stress hormone — has also been found to cause changes in certain cancer cells, specifically prostate and breast cancer, in ways that makes them resistant to apoptosis (cell death).
How chronic inflammation can lead to cancer
https://www.mdanderson.org/publications/focused-on-health/may-2014/inflamation-cancer-diet.html

August 7, 2015
Chronic inflammation caused by disease or exposure to dangerous chemicals has long been linked to cancer, but exactly how this process takes place has remained unclear.
Timing of inflammation determines whether potentially cancerous mutations may arise.A new study from MIT reveals one reason why people who suffer from chronic inflammatory diseases such as colitis have a higher risk of mutations that cause cancer. The researchers also found that exposure to DNA-damaging chemicals after a bout of inflammation boosts these mutations even more, further increasing cancer risk.
This means that emotional stress can both contribute to the development of cancer and reduce the effectiveness of treatments.
DNA Damage and Oxidative Stress in Human Disease
This special issue contains eight papers, covering several aspects of the implication of DNA damage and oxidative stress. Three papers focus on brain, colorectal, and skin cancers and one paper focuses on the genomic stability and oxidative stress in the cancer-predisposing genetic syndrome ataxia telangiectasia. One paper discusses the implication of nutrients in genomic stability in cell cultures. Two papers discuss the effect of a vitamin and a neuroprotectant on diabetes and traumatic brain injury, respectively. Another paper focused on the impact of autophagy on idiopathic pulmonary fibrosis.
“The vitamin D receptor (VDR) gene polymorphisms in Turkish brain cancer patients” by B. Toptas et al. provides evidence for the first time that the risk of meningiomas might be related to polymorphisms in the nuclear receptor of vitamin D, an important factor for the regulation of cell division and proliferation. In “Oxidative stress in the pathogenesis of colorectal cancer: cause or consequence?” M. Pere reviews the interplay between the several risk factors that have been implicated in colorectal cancer, a very common type of cancer in Western countries, which has a complex etiology. N. C. Jenkins and D. Grossman show, in “Role of melanin in melanocyte dysregulation of reactive oxygen species,” that the presence of melanin in the skin appears to be a double-edged sword: it protects melanocytes as well as neighboring keratinocytes in the skin through its capacity to absorb UV radiation, but its synthesis in melanocytes results in higher levels of intracellular ROS that may increase melanoma susceptibility.
L. B. Ludwig et al. provide that ionizing radiation is more efficient than bleomycin to induce chromosomal instability in ataxia telangiectasia patients and that this instability is not related to a systemic increase in oxidative stress. In “The influence of micronutrients in cell culture: a reflection on viability and genomic stability,” A. L. V. Arigony et al. addresse the effect of several vitamins and minerals by reviewing their role in metabolic routes related to DNA homeostasis. The paper presents lines of evidence whether while in deficiency or excess in cell culture the micronutrients reviewed can reduce or increase the level of DNA damage and influence cell proliferation and viability. Finally, the authors advocate which nutrients should deserve more attention in future studies focusing on the increase of genomic stability and cell fitness under culture conditions.
“Vitamin C intake reduces the cytotoxicity associated with hyperglycemia in prediabetes and type 2 diabetes” by S. I. R. Franke et al. compares the levels of vitamin C intake, which is among the most abundant antioxidants obtained from diet, with the levels of markers of hyperglycemia, DNA damage, and cytotoxicity in subjects with type 2 diabetic or with risk of developing the disease. The authors observe that vitamin C intake slightly higher than the dietary recommendation for healthy individuals can be beneficial to the subjects by preventing the cell death of white blood cells that have been reported in the literature to be associated with diabetes complications.
In “Therapeutic time window for edaravone treatment of traumatic brain injury in mice,” K. Miyamoto et al. deal with the edaravone administration postcontrolled cortical impact (CCT) resulting in a significant reduction in the injury volume and oxidative stress. These findings suggest that edaravone could prove clinically useful to ameliorate the devastating effects of traumatic brain injury (TBI). “Self-eating: friend or foe? The emerging role of autophagy in idiopathic pulmonary fibrosis,” by G. A. Margaritopoulos et al. highlights some key issues regarding the process of autophagy and its possible association with the pathogenesis of idiopathic pulmonary fibrosis.
Advances in molecular biology and bioinformatics are allowing researchers to gain an increased understanding of the function and regulation of genes and to identify pathways that are affected. Currently, the search for biomarkers related to disease is gaining increasing attention and especially biomarkers for oxidative stress and DNA damage became more and more valuable instruments for unraveling disease pathogenesis and facilitating prediction, prevention, and treatment of diseases.